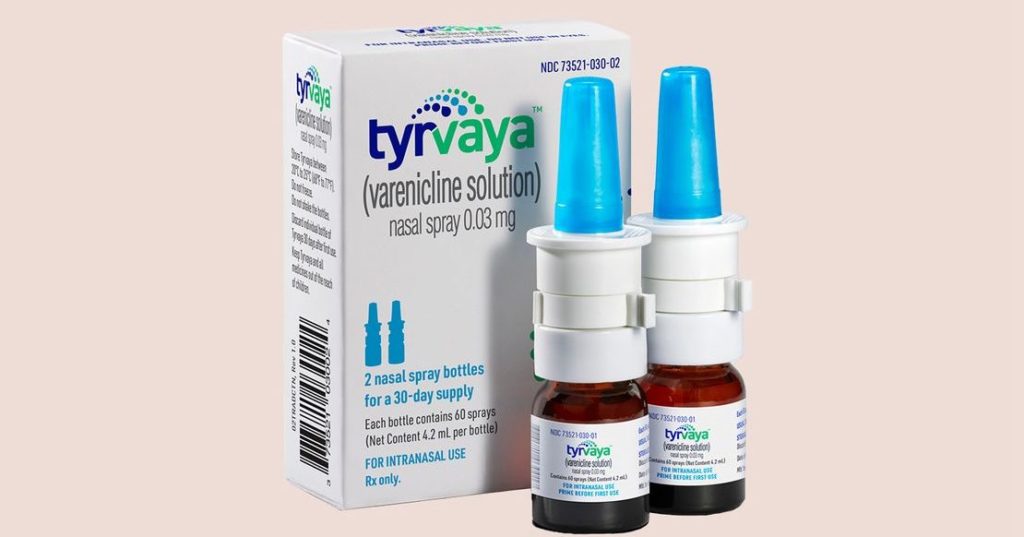
Tyrvaya (varenciline), manufactured by Princeton, New Jersey–based drugmaker Oyster Point Pharma, is the first nasal spray to treat dry eye disease has recently won approval from the US Food and Drug Administration (FDA).
This marks a significant development for patients suffering from moderate-to-severe dry eye. Sprayed twice daily into the nostrils, 0.03-mg varenciline solution (Tyrvaya) improves signs and symptoms of dry eye disease. It provides an alternative to the immunomodulators currently available as prescription treatments.
Varenicline is a safe and effective way to help relieve dry eye disease symptoms for those who have not been able find relief with artificial tears or if they need more than the typical use of 3-4 times per day.
Tyrvaya harnesses cholinergic activity (promoting parasympathetic nervous system processes) to transport medication through the mucous membranes to the trigeminal nerve pathways and into the ophthalmic nerve, to stimulate the nicotinic receptors housed there, which then causes natural tears to be created that help relieve symptoms of dry eyes.
The spray works in as little as 14 days, and doesn’t irritate eyes. In 47% of patients who were given varenicline instead of a placebo eye drops, Schirmer test scores increased by 10 mm within just one month.
Almost all patients who took varenicline had some sneezing, but almost no ocular side effects. There were no reports of burning or stinging; instead some patients experienced some coughing and throat irritation.